Humans
Unmet human pain and wound care need
At present there is no commercially available product for humans that can be sprayed onto wounds in emergency situations to promptly alleviate pain, minimise bleeding, provide antisepsis and create a protective barrier.
Demonstrated commercial success
Originating through the Medical Ethics Group of Companies, Medi-Solfen Pty Ltd has been carved out to develop our technology aiming to prevent pain and minimise suffering associated with wounds in humans.
With the demonstrated commercial success of our lead product, Tri-Solfen®, for animals. Data from these animal studies suggest that a sterile formulation, Medi-Solfen®, may be equally safe and effective for use on humans. A multi-function topical formulation, Medi-Solfen® has been developed from our platform technology, that has the potential to revolutionise the acute management of open wounds in humans, to alleviate suffering, to reduce the risk of blood loss and infection and improve recovery, in the same manner that Tri-Solfen® has done in animals.
Phase 1 human Laceration studies of Medi-Solfen®, have recently commenced with the successful treatment of the 3 patient cohorts, in Kyiv Ukraine.

Chronic wound care
Medi-Solfen® has the potential to treat chronic wounds such as venous leg ulcers, diabetic foot ulcers and pressure ulcers
Chronic wounds affect millions of people globally, which is expected to rise significantly due to the increasing proportion of elderly patients living longer. There are three main types of chronic wounds; venous leg ulcers, diabetic foot ulcers and pressure ulcers.
Cleansing chronic wounds of local barriers, such as dead tissue, is known as debridement and is an essential process to facilitate efficient healing. It has been shown that faster and more complete healing is achieved with more frequent debridement.
However, debridement of chronic wounds is not well tolerated and patients will often ask clinicians to stop before the procedure is completed, or avoid the procedure, because of the pain and discomfort. This pain also extends beyond completion of the procedure.
Patients with leg ulcers report pain to be the worst aspect of having an ulcer. Chronic pain is highly complex, may extend over months or years, and has enormous implications for a patient’s quality of life, causing decreased activities of daily living, sleep disturbance, reduced mobility and social withdrawal.
It has been shown that when patients with leg ulcers feel an ease or absence of pain they interpret it as a positive sign of wound healing, which can make them feel hopeful and optimistic.
Supported by trial data from our approved animal product (Tri-Solfen®), Medi-Solfen Pty Ltd is initiating the regulatory approval process, for Medi-Solfen®, for a number of indications in humans.
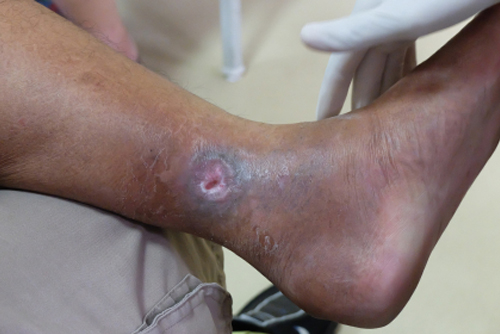
1 in 500 people suffering
Venous leg ulcers are estimated to affect around 1 in 500 people in the UK, although they become much more common with age. It's estimated that around 1 in 50 people over the age of 80 has one.
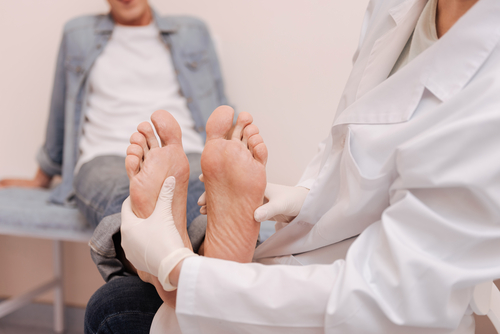
Up to 10% of diabetics
422 million adults have diabetes. Up to 10% of them will develop a diabetic foot ulcer at some point.
Supporting studies and references
Cost of wound care
Economic pressure and challenges to prove efficacy in a world where populations and social burden is growing, means healthcare payors are increasingly asking for financial justification for treatments. Medi-Solfen® aims to reduce both the financial and personal costs relating to chronic wound care.
The following scientific publications and reference materials support our efforts towards the cost of wound care:
The costs of skin breakdown and ulceration in the UK
Read more
SkinBreakdown
Chronic wounds represent a significant burden in the UK – to patients and to the healthcare system. With proper diagnosis and treatment, much of this burden is avoidable. The purpose of this paper is to provide an estimate of the annual cost of chronic wounds to the NHS. There are around 200,000 individuals in the UK at any time with a chronic wound – many more new wounds develop annually. The cost to the NHS of caring for these patients is conservatively estimated at £2.3bn to £3.1bn per year (at 2005/06 prices) – around 3% of total estimated 2005/06 expenditure on health (£89.4bn).
See full PDF
Close
Making the case for cost-effective wound management – an expert working group review
Read more
International Consensus
Clinicians who treat patients with wounds need access to the resources that will enable them to deliver the best and most appropriate treatments. With economic constraints on healthcare budgets, in addition to challenges to prove efficacy, budget holders and payors are increasingly asking for financial justification for the provision of treatment. Clinicians therefore need to know how to provide such justification to ensure continued provision of appropriate wound management services, including the implementation of service improvements and new technologies.
See full PDF
Close
Venous leg ulcer management in clinical practice in the UK: costs and outcomes
Read more
International Wound Journal
The aim of this study was to estimate the patterns of care and annual levels of health care resource use attributable to managing venous leg ulcers (VLUs) in clinical practice by the UK’s National Health Service (NHS) and the associated costs of patient management. This was a retrospective cohort analysis of the records of 505 patients in The Health Improvement Network (THIN) Database. Patients’ characteristics, wound-related health outcomes and health care resource use were quantified, and the total NHS cost of patient management was estimated at 2015/2016 prices. Overall, 53% of all VLUs healed within 12 months, and the mean time to healing was 3⋅0months. 13% of patients were never prescribed any recognised compression system, and 78% of their wounds healed. Of the 87% who were prescribed a recognised compression system, 52% of wounds healed. Patients were predominantly managed in the community by nurses with minimal clinical involvement of specialist clinicians. Up to 30% of all the VLUs may have been clinically infected at the time of presentation, and only 22% of patients had an ankle brachial pressure index documented in their records. The mean NHS cost of wound care over 12months was an estimated £7,600 per VLU. However, the cost of managing an unhealed VLU was 4.5 times more than that of managing a healed VLU (£3,000 per healed VLU and £13,500 per unhealed VLU). This study provides important insights into a number of aspects of VLU management in clinical practice that have been difficult to ascertain from other studies and provides the best estimate available of NHS resource use and costs with which to inform policy and budgetary decisions.
See full PDF
Close
Health economic burden that wounds impose on the National Health Service in the UK
Read more
BMJ Open
This study estimated the health outcomes, resource implications and associated costs attributable to managing wounds in 2012/2013 using real world evidence obtained from The Health Improvement Network (THIN) database (a nationally representative database of clinical practice among >11 million patients registered with general practitioners in the UK).
See full PDF
Close
Medi-Solfen®
Pain and wound healing the direct correlation
Patients living with a chronic wound are often subjected to dressing changes, which can exacerbate their pain and dressing removal is considered to be the most painful aspect of the dressing procedure. This is particularly problematic where a dressing has stuck to the wound or removal of a dressing has torn the skin. Pain at dressing changes can also be evoked by the debridement of slough and necrotic tissue, the application of antiseptics and the use of wound cleansing procedures.
Optimising wellbeing in people living with a wound: an expert working group review
Read more
International consensus
People ‘at risk’ of, or those living with a wound face major changes in their everyday lives and
need to integrate a number of treatment-related procedures that may be difficult to adopt long
term and conflict with existing lifestyles, priorities and behaviours
See full PDF
Close
Topical agents or dressings for pain in venous leg ulcers
Read more
The Cochrane Collaboration
Venous leg ulcers affect up to 1% of people at some time in their lives and are often painful. The main treatments are compression bandages and dressings. Topical treatments to reduce pain during and between dressing changes are sometimes used.
See full PDF
Close
Pain at wound dressing-related procedures: a template for assessment
Read more
World Wide Wounds
This paper presents a simple, practical framework to assess a patients experience of pain at wound dressing-related procedures that has been inspired by the World Union of Wound Healing Societies’ consensus document.
See full PDF
Close
Pain and stress as contributors to delayed wound healing
Read more
Upton D & Solowiej K
It is possible that patients who suffer from acute and chronic wounds can interpret wound pain as a stressor. It is known from previous research that stress can delay wound healing; however, little is known about the influence of pain in this relationship. This review explores the literature surrounding the relationship between stress, pain and delayed healing of acute and chronic wounds. Many studies have demonstrated the impact of stress on the healing of biopsy, surgical and chronic wounds and there is a range of medical, psychological and social interventions that may reduce both pain and stress and consequently speed up wound healing.
See full PDF
Close
Pain and wound healing in surgical patients
Read more
Annals of Behavioral Medicine
Human and animal laboratory studies have shown that stress delays healing of standardised punch biopsy wounds. This 5-week prospective study of 17 women who underwent elective gastric bypass surgery addressed the association between postsurgical pain intensity and subsequent healing of a standard 2.0-mm punch biopsy wound.
See full PDF
Close
European Wound Management Association (EWMA)
Understanding wound pain and trauma, the theory of pain, and pain at wound dressing changes: a guide to management.
See full PDF
Close
Acute and chronic pain: where we are and where we have to go
Read more
Acute and chronic pain: where we are and where we have to go
In recent years, increasing attention has been focused on the treatment of acute and chronic pain with a considerable number of publications about it. Nevertheless all the attention focused on it, the evidence of pain treatments is still unfolding, and occasionally conflicting. Hence it is still necessary that we point out our research efforts in trying to obtain a better understand of pathophysiology of pain and of real efficacy and safety of acute and chronic pain treatments.
See full PDF
Close
Exploring the effects of pain and stress on wound healing
Read more
Clinical Management Extra
Wound-related pain is complex, involving a multitude of physiological and psychological factors, such as emotional state, culture, personality, meanings, and expectations. The impact of pain on the individual can contribute to stress and compromise quality of life. The purpose of this article is to review the relationships among pain, stress, and wound healing.
See full PDF
Close
Wound bed preparations
Wound bed preparation is a comprehensive approach to wound management that focuses on optimising conditions at the wound bed to encourage normal endogenous processes of healing. It is based on an understanding of the underlying molecular processes of the wound and is in a continuous state of evolution as it incorporates and responds to new information about and understanding of cellular mechanisms.
The following scientific publications and reference materials explain the concept of TIME wound healing about the relevance of wound bed preparation to diabetic wounds and burns:
What have we learned in the past 10 years?
Read more
Extending the TIME concept
The TIME acronym (tissue, infection/inflammation, moisture balance and edge of wound) was first developed more than 10 years ago, by an international group of wound healing experts, to provide a framework for a structured approach to wound bed preparation; a basis for optimising the management of open chronic wounds healing by secondary intention. However, it should be recognised that the TIME principles are only a part of the systematic and holistic evaluation of each patient at every wound assessment. This review, prepared by the International Wound Infection Institute, examines how new data and evidence generated in the intervening decade affects the original concepts of TIME, and how it is translated into current best practice.
See full PDF
Close
European Wound Management Association (EWMA)
The last two decades have focused on improving the healing rates of patients with a wide range of chronic wounds. It is now realistic to expect that, with evidence-based care, many wounds will heal uneventfully within a reasonable timeframe. Despite overall improvements, however, there remains a small but significant proportion of chronic wounds that fail to heal even with the highest standard of care. The management of these wounds has therefore come under scrutiny and attention has turned to the factors that influence their healing and to the preparation of the wound bed.
See full PDF
Close
New concepts and scientific applications
Read more
Wound healing and TIME
This article reviews recent thinking about the relevance of wound bed preparation to diabetic wounds and burns, presents adjustments to the tissue, infection/ inflammation, moisture imbalance, epidermis/edge (TIME) paradigm of wound management to reflect greater understanding concerning migration of the epidermal edge, and provides an extension of the concept to include wound assessment. Finally, it asks whether TIME should now be put to the test. The field of wound management is lacking in robust data, but now that a more systematic approach to wound care exists, perhaps a more systematic approach to gathering the evidence should also be attempted.
See full PDF
Close
Future opportunities
Future near term opportunities for Medi-Solfen®, include the treatment of traumatic wounds caused by surgical procedures, and mass trauma situations such as accidents, military conflict and natural disasters.
Future human indications include:
- Chronic wound care
- Immediate wound treatment
- Medical clinics
- Defence forces
- Emergency services
- Natural disaster situations
- Post-surgery wound care
- Over-the-counter first aid treatments




